Novel Energetic Materials Research:
Application of high pressure significantly alters the interatomic distance and thus the nature of intermolecular interaction, chemical b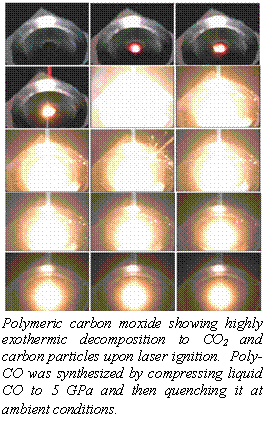 onding, molecular configuration, crystal structure, and stability of solid. With modern advances in high-pressure technologies, it is feasible to achieve a large (often up to aseveral-fold) compression of lattice, at which condition material can be easily forced into a new physical and chemical configuration. The high-pressure thus offers enhanced opportunities to discover novel materials, both stable and metastable ones, and to tune exotic properties in a wide-range of atomistic length scale, substantially greater than (often being several orders of) those achieved by other thermal (varying temperatures) and chemical (varying composition or making alloys) means. The thrust materials of interest in our research include:
onding, molecular configuration, crystal structure, and stability of solid. With modern advances in high-pressure technologies, it is feasible to achieve a large (often up to aseveral-fold) compression of lattice, at which condition material can be easily forced into a new physical and chemical configuration. The high-pressure thus offers enhanced opportunities to discover novel materials, both stable and metastable ones, and to tune exotic properties in a wide-range of atomistic length scale, substantially greater than (often being several orders of) those achieved by other thermal (varying temperatures) and chemical (varying composition or making alloys) means. The thrust materials of interest in our research include:
- High Energy Density Materials: the materials made of low z extended solids in three-dimensional network structures such as cubic gauche-nitrogen and extended metal hydrides:
- Energetic Materials: including high explosives (such as nitromethane, TNT, RDX, etc.); implovised explosive materials (such as hydrogen peroxide and ammonium nitrates); detonation products including carbon dioxide, water, nitrogen, carbon, and their mixtures
- Superhard Solids: the materials like diamond and cubic boron nitrides as
- Functional Intermetallics: of d- and f-electron metals in ordered, disordered and layered forms.
This project has been supported by:
- DHS-Alert (2013-ST-061-ED0001 and 2008-ST-061-ED0001) for Chemical and Shock Mitigation of Nonconventional Explosives Threats
- DTRA (HDTRA1-12-01-0020): High-Energy-Density Monolithic Organometallic Solid
- LANL (ASM-SUB, 84461-SOL-10): Thermochemical properties of explosives and detonation products.
References: on high energy density materials
- Phase diagram and transformation of iron pentacarbonyl to nm layered hematite and carbon- oxygen polymer under pressure, YoungJay Ryu, Minseob Kim, and Choong-Shik Yoo, Scientific Reports (2015) DOI:10.1038/srep15139
- Pressure-induced symmetry lowering transition in dense nitrogen to layered polymeric nitrogen (LP-N) with colossal Raman intensity, D. Tomasino, M. Kim, J. Smith, and C. S. Yoo, Phys. Rev. Lett. 113, 205502 (2014)
- Novel 2D and 3D Extended Solids and Metallization of Compressed XeF2, Minseob Kim, Mathew Debessai, and Choong-Shik Yoo, Nature Chem. 2, 784 (2010).
- High Energy Density Solid, Choong-Shik Yoo, in Shock Compression of Condensed Matter-2009, edited by M.L. Evert, et al., Part I, p 11 (AIP Press, 2009).
- Extended Networks of Nitrogen: Reddish Amorphous- and Transparent Cubic Gauche Nitrogen Polymers, Magnus J. Lipp, Jae-Hyun Klepeis, Bruce Baer, Hyunchae Cynn, William J. Evans, Valentin Iota, and Choong-Shik Yoo, Phys. Rev. B. 76, 14113 (2007).
- Pressure-Induced Disproportion of Carbon Monoxide to Carbon Dioxide and Energetic Lactonic Polymer, W.J. Evans, M.J. Lipp, C. S. Yoo, H. Cynn, J. Herbert, R.J. Maxwell, M.F. Nicol, Chem. Mater. 18, 2520 (2006).
- High-Energy-Density Extended CO Solid, M.J. Lipp, W. J. Evans, B. Baer, and C.S. Yoo, Nature Materials 4, 211 (2005)
on conventional and improvised energetic materials
- Phase Transitions in I2O5 at High Pressures: Raman and X-ray Diffraction Studies, Minseob Kim and Choong-Shik Yoo, J. Chem. Phys. in review (2015).
- Phase Diagram of Ammonium Nitrate, Mihindra Dunuwille and Choong-Shik Yoo, J. Chem. Phys. 139, 214503 (2013).
- “Stubborn” Triaminotrinitrobenzene (TATB): Unusually high chemical stability of a molecular solid to 150 GPa, Alistair Davidson, Ranga P. Dias, Dana M. Dattelbaum, and Choong-Shik Yoo, J. Chem. Phys. 135, 174507 (2011)
- Pressure induced isostructural metastable phase transition of ammonium nitrate, Alistair J. Davidson, Raja S. Chellappa, Dana M. Dattelbaum, and Choong-Shik Yoo, J. Phys. Chem. C 115, 11889 (2011).
- Phase Transition and Chemical Decomposition of Hydrogen Peroxide and its Water Mixtures under High Pressures, Jing-Yin Chen, Minseob Kim, Choong-Shik Yoo, Dana Dattelbaum, and Steve Sheffield, J. Chem. Phys 132, 1 (2010).
- High Stability of Single Wall Carbon Nanotube in Quasi-Hydrostatic Conditions, Jing-Yin Chen, Minseob Kim, and Choong-Shik Yoo, Chem. Phys. Lett. (2009) in print.
- High Pressure-induced Phase Transitions in Pentaerythritol: X-ray and Raman Studies, Z. A. Dreger, Y. M. Gupta, C.-S. Yoo, and H. Cynn, J. Phys. Chem. B109, 2258 (2005)
- A Quantum Mechanical Molecular Dynamics Study of Binary Collisions of Pentaerythrol Tetranitrate (PETN): Its Correlation to Shock Sensitivity, C. J. Wu, Francis H. Ree and Choong-Shik Yoo,Propellants, Explosives, Pyrotechnics 29. 296 (2004).
- Anisotropic Shock Sensitivity and Detonation Temperature of Pentaerythrol Tetranitrate (PETN) Single Crystal, C.S. Yoo, N.C. Holmes, C.P. Souers, C.J. Wu, F.H. Ree, J.J. Dick, J. Appl. Phys., 88, 1 (2000).
- Equation of State, Phase Transition, Decomposition of b-HMX(Octahydro-1,3,5,7-Tetranitro-1,3,5,7-Tetrazocine) at High Pressures, C.S. Yoo, and H. Cynn, J. Chem. Phys, 111, 10229 (1999).
- Phase Transition and Decomposition of 90 % Hydrogen Peroxide at High Pressures, H. Cynn, C.S. Yoo, and S.A. Sheffield, J. Chem. Phys. 110, 6836 (1999).
Dynamic response of reactive materials:
Reactive materials such as metal powders, thermite mixtures, mixed composites, and nano-energetics are an emerging class of energetic materials, whose thermochemical responses are very different from hydrocarbon-based energetic materials. In order to evaluate the performance and reliability of reactive materials, one needs to know the dynamic responses over a wide range of time scales; for example, ms for intermetallic reactions, ms-ms for metathesis reactions, ms for metal combustion, and ns for detonation. Hence, the goal of this project is to understanding dynamic responses of reactive materials, particularly, via resolving the structural and chemical evolution of reactive materials under rapidly propagating, exothermic reactions, using various time-resolved spectroscopic and diffraction technologies. The development of new enabling technologies are also of interest to this project.
 Recently, we have developed time-resolved synchrotron x-ray diffraction at the APS, utilizing a large 2D x-ray detector and a fast running x-ray chopper. This new technique has been applied to get real-time structural information during metal combustions and intermetallic reactions, as shown in the figure. It shows the time-resolved x-ray diffraction patterns obtained during Zr combustion with a 30 ms time resolution. This result reveals several significant facts: (i) the combustion occurs from molten phase of Zr – the first direct evidence observed. (ii) The combustion process is substantially more complex than what we have typically assumed previously (Zr + O2 -> ZrO2). It undergoes a series of transformations from a-Zr -> b-Zr -> liq-Zr -> ZrO -> Zr3O -> ZrO2, many of these transformations are exothermic, thus, important for understanding the energetics of Zr combustion. (iii) quantitative chemical – time information regarding the combustion. Note that these are critical data to develop/validate thermochemical models. In fact, many thermochemical libraries currently used have a limited data basis for reactive materials and incomplete descriptions of metal combustions.
Recently, we have developed time-resolved synchrotron x-ray diffraction at the APS, utilizing a large 2D x-ray detector and a fast running x-ray chopper. This new technique has been applied to get real-time structural information during metal combustions and intermetallic reactions, as shown in the figure. It shows the time-resolved x-ray diffraction patterns obtained during Zr combustion with a 30 ms time resolution. This result reveals several significant facts: (i) the combustion occurs from molten phase of Zr – the first direct evidence observed. (ii) The combustion process is substantially more complex than what we have typically assumed previously (Zr + O2 -> ZrO2). It undergoes a series of transformations from a-Zr -> b-Zr -> liq-Zr -> ZrO -> Zr3O -> ZrO2, many of these transformations are exothermic, thus, important for understanding the energetics of Zr combustion. (iii) quantitative chemical – time information regarding the combustion. Note that these are critical data to develop/validate thermochemical models. In fact, many thermochemical libraries currently used have a limited data basis for reactive materials and incomplete descriptions of metal combustions.
WSU is a leading institute for high-pressure research both in static and dynamic research, with a wide range of enabling capabilities such as laser-heated DAC, dynamic-DAC, micro impactor, laser ablator, and various gas guns – all coupled with cutting-edge time-resolved spectroscopy, high-speed microphotography, time-resolved spectro-pyrometry, and the third-generation synchrotron x-ray source at the APS.
This research has been in support of:
- ARO (W911NF-14-1-0233): Dynamic Responses of Reactive Metal Alloys
- DHS (2013-ST-061-ED0001): ALERT Center for Energetic Materials
- DARPA (W911NF-09-C-0033): Reactive Materials Structure: Chemical Performance (co-PI with four others, Completed, 2009-2011)
References:
- Time-resolved x-ray diffraction of reactive solids under dynamic loadings, Choong-Shik Yoo, J. Phys.: Conf. Ser., in print (2015)
- Probing Dynamic Crystal Growth of Compressed Hydrogen using Dynamic-DAC, Time-Resolved Spectroscopy and High Speed Microscopy, Dane Tomasino and Choong-Shik Yoo, J. Phys. Con. Ser. 500, 032019 (2014).
- Time-resolved X-ray Diffraction Across Water-Ice VI/VII Transformations using Dynamic-DAC, Jing-Yin Chen, Minseob Kim, Choong-Shik Yoo, and William Evans, J. Phys. Conf. Ser. 500, 142006 (2014).
- Solidification and Crystal Growth of Highly Compressed Hydrogen and Deuterium: Time-Resolved Study under Ramp Compression in Dynamic-Diamond Anvil Cell, Dane Tomasino and Choong-Shik Yoo, Appl. Phys. Lett. 103, 061905 (2013).
- Dynamic Responses of Reactive Metallic Structures under Thermal and Mechanical Ignitions, Haoyan Wei and Choong-Shik Yoo, J. Mater. Res. 27, 2705 (2012).
- Kinetics of Small Single Particle Combustion of Zirconium Alloy, Haoyan Wei and Choong-Shik Yoo, J. App. Phys. 111, 023506 (2012).
- Oxygen-Diffusion Limited Metal Combustions in Zr, Fe and Ti Foils: Time-Resolved X-ray Diffraction Studies, Haoyan Wei, Jing-Yin Chen and Choong-Shik Yoo, J. App. Phys. 111, 063528 (2012).
- Time- and angle-resolved x-ray diffraction to probe structural and chemical evolution during Al-Ni intermetallic reactions, ChoongShik Yoo, Haoyan Wei, Jing-Yin Chen, Guoyin Shen, Paul Chow, and Yuming Xiao, Rev. Sci. Instrum. 82, 113901 (2011).